 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|
Further Observations of a Possible Biological Alteration of Radioactive Decay |
|
|
|
by Fungi: |
|
|
|
Second Round Testing |
|
|
|
N.A. Reiter |
|
|
|
Dr. S.P. Faile |
|
|
|
28 August, 2002 |
|
|
| Background and Objective: |
|
|
| In
early August, 2002, we conducted an initial experiment that appeared to
indicate an alteration of the radioactive decay of thorium by a unique
fungal culture. A second round of experiments was designed and
performed, with further interesting results. An additional source of
nuclear material was used - uranium in the form of uranyl acetate. |
|
|
| Procedure: |
|
|
| For
this experiment, we use the same semi-dried fungal matrix (the S.P.
Faile "Fort Hill" fungus) from our first round experiment. For
detection of radiation, we once again use our Baird Atomic 916 lab
Geiger counter. With voltage set for 900V, we confirm that the
background count rate in the lab appeared to typically run between 10
and 30 CPM. |
|
|
| Our
second experiment was again performed at our lab location in the
Toledo, Ohio area. The ambient consisted of a typical air conditioned
office / lab atmosphere, with typical temperature running about 24oC
+/- 2oC. |
|
|
| Four
6 inch square plastic tubs were washed thoroughly with methanol, then
dried. Room temperature unflavored soy milk was added to each, 150 ml
worth. To the first tub, our control, an additional 20 ml of soy milk
was added, along with 20 ml of a 1:1 solution of saturated thorium
nitrate in distilled water, diluted with an equal volume of distilled
water. |
|
|
| To
the second tub, we add 20 ml of soy milk in which 1 gram of our
semi-dried fungal matrix was minced and mixed. We then add 20 ml of the
thorium nitrate:H2O solution. |
|
|
| Tub
#3 is filled with an identical volume of soy milk as tub #1, however
instead of the thorium nitrate, we add 20 ml of a 1:1 mixture of
saturated uranyl acetate in distilled water, diluted with distilled
water. This tub constituted out uranium containing control. |
|
|
| Tub
#4 is prepared similarly to the cultured tub #2, except for the
substitution of the uranyl acetate solution for the thorium nitrate. |
|
|
| All
four of the control and cultured tubs were shaken to disperse the
solutions. We note that within a few seconds, a slight curdling of the
soy milk was seen in all tubs. |
|
|
| One
difference in protocol was made, within this experiment. We start all
tubs out with their plastic lids placed over them (non-sealed, but a
more solid diffusion barrier than the paper towel covers used in the
first experiment.) |
|
|
| The
Geiger counter was used to take count rates for all tubs, beginning
immediately after preparation, and then periodically for the next 14
days. This data was recorded, and is presented herein. Our readings
were taken by holding the GM tube vertically over the soymilk
solutions, at tub center and at tub corners. Max and min count rates
were recorded. We also record our visual records of the physical
properties for both cultured and control tubs. |
|
|
| Results: |
|
|
|
Hours |
|
Tub1 |
|
Tub2 |
|
Tub3 |
|
Tub 4 |
|
Notes |
|
|
|
(Th-control) |
|
(Th-culture) |
|
(U control) |
|
(U culture) |
|
All curdled |
|
|
|
0 |
|
140-180 |
|
160-180 |
|
180-200 |
|
160-180 |
|
|
|
4 |
|
160-180 |
|
160-180 |
|
180-200 |
|
160-180 |
|
|
|
24 |
|
180-220 |
|
220-300 |
|
200-240 |
|
220-260 |
|
|
|
30 |
|
180-240 |
|
260-320 |
|
200-260 |
|
240-300 |
|
|
|
48 |
|
220-240 |
|
240-360 |
|
300-360 |
|
240-300 |
|
Still no mat formation ?replace lids w/ towels |
|
|
|
|
|
72 |
|
220-260 |
|
280-400 |
|
420-500* |
|
260-340 |
|
Green mat starting for #2 and #4 |
|
|
|
|
|
96 |
|
220-260 |
|
280-360 |
|
500-800 |
|
240-320 |
|
|
|
120 |
|
240-380 |
|
280-320 |
|
1000-1100 |
|
220-240 |
|
|
|
144 |
|
280-380 |
|
200-280 |
|
1000-1100 |
|
200-240 |
|
|
|
168 |
|
260-340 |
|
180-240 |
|
800-1000** |
|
320-400 |
|
#3 still wet and jelly-like. |
|
|
|
|
|
192 |
|
260-340 |
|
180-240 |
|
800-900 |
|
360-500*** |
|
|
|
216 |
|
300-360 |
|
280-320 |
|
800-900 |
|
320-440 |
|
|
|
240 |
|
280-340 |
|
240-280 |
|
700-900 |
|
380-480 |
|
|
|
264 |
|
240-320 |
|
240-280 |
|
700-900 |
|
360-440 |
|
|
|
264 |
|
320(max) |
|
280(max) |
|
900(max) |
|
440(max) |
|
Add foil to GM tube aperture |
|
|
|
|
|
312 |
|
|
|
336 |
|
|
| Data
notes: * Areas or colonies of "rogue" mold formed ? reddish brown
blobs. **Reddish colonies look dead at this point. ***Secondary growth
of a whitish mold forming. |
|
|
 |
|
|
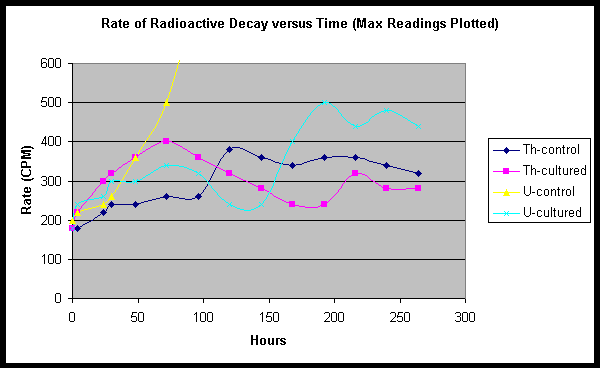
From
the raw data as well as plotted values, we find evidence of a
potentially complex set of phenomena. In our first experiment, a
clearly defined difference in radioactive decay rate with time was
noted between cultured and non-cultured tubs. In this experiment, we
again find drastic differences in performance, however the rising and
falling of nuclear decay count rates seem to point to multiple
influences. In general, however, we may be able to discern
relationships in a clearer manner by comparing pairs of samples.If one
compares Th tubs only, we find that the relationship between cultured
and control seems to resemble results from our first experiment. For
our cultured Th tub, CPM is seen to rise strongly, and faster than the
control tub, peaking at a high count rate, then diminishing. Our
control Th tub rises slowly for several days, until visible colonies of
mold are seen to form on the soy milk. At this point in time, the CPM
begins to rise abruptly, but then drops off at a slower rate after
peaking.
If one compares the uranyl acetate doped culture with the
Th doped culture, one also finds a similarity of curves at least for
about the first 160 hours.
Later peaks and dips in CPM may need
better explanation, however we do observe a likely correlation to the
on-set of secondary fungi species.
The maverick component of this
experiment is, without a doubt, tub #3, the uranyl acetate containing
control tub. We find that the soaring CPM does correspond generally
with the appearance of several "blob" or isolated colonies of a reddish
brown mold. Following the death/darkening of these blobs, the whole
surface of the soy milk became shiny with a clear gelatinous residue.
SPF believes that the rogue reddish brown colonies were the Fort Hill
fungus in a mutated form or different culture density. |
|
|
| Other
observations abound. Special attention was paid to the distribution of
min and max CPM readings in all tubs, however for the two "control"
tubs, in which rogue fungi took hold, we find that the distribution of
readings appeared to remain steady, and be stronger in the tub center,
whether or not a fungal colony was growing over it or not. This hints
at a fairly uniform distribution of the radioactive components in the
soy milk matrix. |
|
|
| We
also desired to try to isolate the primary emission product we were
seeing. A simple test was performed in which we placed a single layer
of heavy gauge kitchen aluminum foil over the Geiger Mueller tube
aperture and took a second set of max CPM readings for each tub. All
four readings remained un-changed, thus suggesting that the decay mode
we are dealing with, at least in the later stages of the experiment, is
primarily gamma or hard X-ray emission, as opposed to alpha decay or
lower energy neutrons. |
|
|
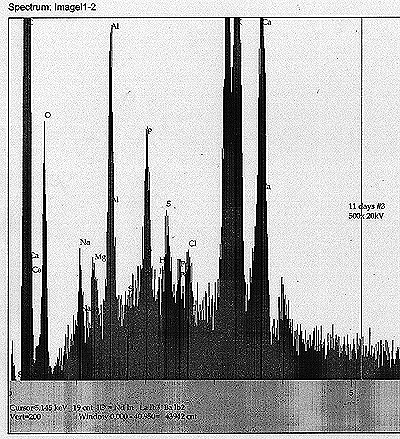
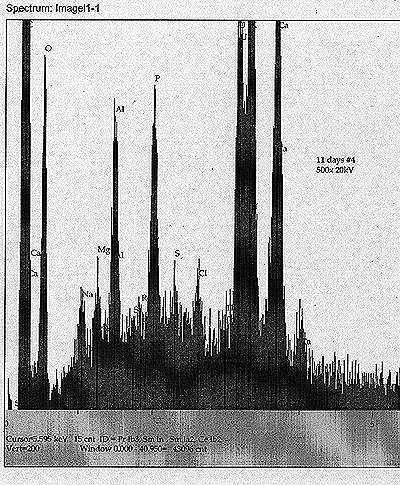
| EDS Results: |
|
|
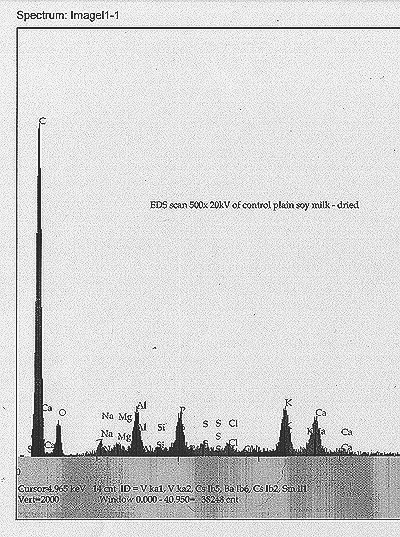
At
T+ 11 days, we procured small smear samples from the soy milk matrix
near the center of each tub. These were dried under a heat lamp, and
then placed into our scanning electron microscope for EDS analysis. Our
intention was to identify any elemental species that could be construed
as anomalous, or as possible transmutation products. To facilitate
this, an EDS scan of dried plain soy milk was also taken as a control.
We find:The soy milk control: high carbon peak, with modest peaks of O,
P, K, Ca and Al (aluminum may be from SEM fixturing). Minor peaks for
Na, Mg, Si, S, and Cl.
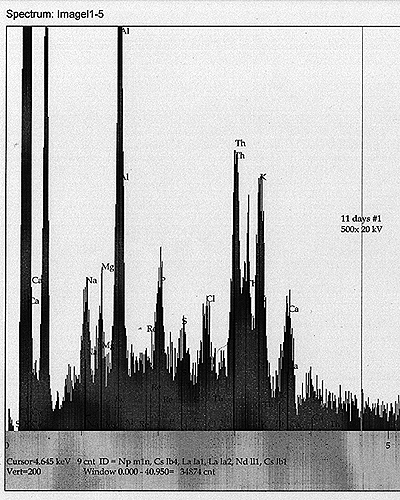
Tub #1 ? Th Control: In addition to the above components, we also see a Th peak and a possible very minor peak for Re.
Tub
#2 ? Th Cultured: In addition to the soy milk components, we see a
minor but apparently real peak for W, as well as a possible peak for Bi.
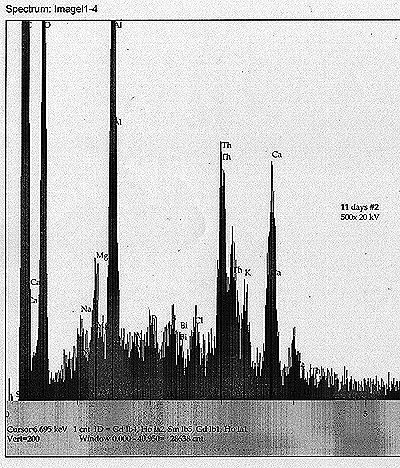
Tub
#3 ? U Control: Most noticeable peak apart from the soy milk components
and U is a peak that appears to correspond to Po ? of about equal
magnitude as the Cl peak nearby. There may exist also a minor Hg peak.
Tub #4 ? U Cultured: Soy milk components, along with U. Also, a minor peak for Th, as well as a very minor peak for Re. |
|
|
| From
these results, we find some preliminary but significant evidence for
the appearance of daughter products and possible unpredicted
transmutation. |
|
|
| See scans shown for each analysis. |
|
|
| Discussion: |
|
|
| From
our second round results, we find further evidence for our basic
premise - that the growth of a fungal culture in a matrix of soy milk
containing a modest amount of radio-isotope appears to cause a
modification of the measured nuclear decay rate of said doped matrix.
Because of the difficulty involved with following the non-visible
growth of molds and fungi in their early stages, we have incomplete
correlation. However, there is little doubt that the effects seen in
the second round of testing corroborate the main observations from the
first round. |
|
|
| We
also, in this round, have acquired some interesting EDS analysis that
appears to show the development of new elemental peaks after eleven
days of growth. In the case of the tub containing the most drastically
altered decay rate, we find a signal for Po that appears to be of a
significant level - about equal to the primary X-ray line for Cl. |
|
|
| In
August, welcomed review of our first report brought to light a proposed
artifact mechanism. It was suggested that nitrogen uptake by the fungi
was being accompanied by an uptake of radon, which would otherwise
normally diffuse away into the nearby atmosphere at a constant rate.
Thus, the overall rate of radioactive emission would be seen to
increase. We are still evaluating this idea, and need to find a better
means to test it. |
|
|
| We
are currently planning a third round of tests, focusing on either the
Th or U compounds, but also looking at the effects of other types of
fungi or bacteria. |
|
|
| We
extend our thanks toward the technical correspondents who have
contributed to this project thus far. Special thanks go to the faithful
members of the Vortex on-line discussion group. |
|
|
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
 |
|






